
September 5, 2024
Thorough Review Of Present And Approaching Anti-obesity Medications
Thorough Testimonial Of Present And Approaching Anti-obesity Drugs NeuroSearch has also reported interim outcomes [9] from a 48-week, open-label, extension trial (TIPO-4) in which 140 patients that completed the 24-week stage IIB trial (TIPO-1) were re-enrolled after approximately 3 months' wash-out. All were at first treated with 0.5 mg tesofensine daily however up-titration to 1.0 mg once daily was allowed the first 24 weeks of the extension research study. The 24-week interim results for those who were previously treated with tesofensine 0.5 mg in TIPO-1 showed a total mean weight-loss of between 13 kg and 14 kg over 48 weeks of therapy. In addition, TIPO-4 confirmed the TIPO-1 results since those clients who were previously treated with placebo lost approximately 9 kg in the first 24 weeks of the TIPO-4 research. The lots of prospects presently being considered recommend that or even more may achieve this lofty purpose. As component of the authorization process, the FDA asked for that Orexigen, thesponsor, perform a cardio safety research to demonstrate that NB-32doesn' t boost major occasions as identified by a non-inferiority hazardratio of less than 1.4. Orexigen enlisted 8,910 obese and overweight topics inan result study, LIGHT, driven by the variety of major cardio eventsincluding non-fatal stroke, non-fatal myocardial infarction, and cardiovasculardeath. The test confirmed that after the 25% and 50% meantime analyses ofevents, the non-inferiority threat proportion was less than 2.0. The sponsor brokethe blind and released secret information halfway via the trial andinvalidated the outcomes prior to the noninferiority risk proportion of 1.4 or lesswas reached, producing a demand to repeat the trial under properly blindedconditions [49]- This is a breakthrough efficiency about registered AOMs that asks the question of what the greatest following top priority is, and whether we have the abilities necessary to properly attain it.
- It's important to conduct such comparisons as it fosters far better understanding of how these treatments run and their possible benefits for individuals.
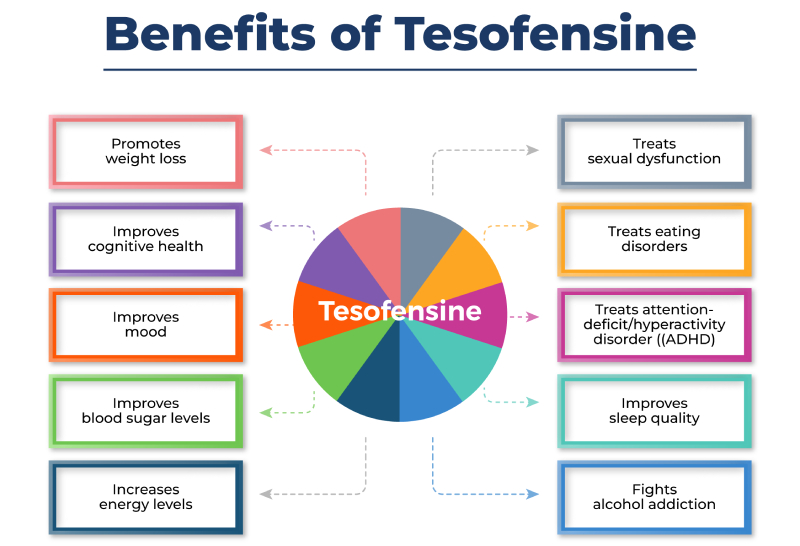
- The performance of tesofensine in lowering body weight and managing appetite, in addition to its safety profile, makes it an exciting candidate for future clinical tests in humans.
- More growth details to glucagon-like peptides has actually been anchored by the improved performance demonstrated for GLP1 co-agonists with GIP or glucagon agonism.
Component Three Future Generation Obesity Treatments
In the last century, the pharmacological administration of obesity has included amphetamines, thyroid hormonal agents, dinitrophenol and numerous medication combinations (rainbow pills) that were withdrawn soon after governing authorization because of major unfavorable effects34 (Table 1). Several centrally acting sympathomimetics such as phentermine, cathine and diethylpropion proceed in short‐term use. A serious awareness throughout a lot of these methods is the typical inability to achieve placebo-adjusted mean weight management above 10% of initial body weight when constantly administered at bearable doses. As greater weight management is accomplished, it is commonly accompanied by different significant acute or persistent negative effects34 (Table 1).Helpful Physiological And Performance Responses To A Month Of Restricted Power Intake In Healthy And Balanced Obese Ladies
Restorative rate of interest has been spurred by monitorings in rats, where neutralization of acyl-ghrelin246, restraint of ghrelin O-acyltransferase (GOAT) as the activating fatty acylation enzyme247 or straight animosity of GHSR248 have actually shown reductions in body weight and food intake. Excessive weight is a quickly expanding condition that arises from a discrepancy betweenfood consumption and energy expense. However, therapy of obesity is hamperedby organic pressures that resist maintenance of fat burning. The length of drugtreatment needed was thought to have to do with 12 weeks, the length of time required tobreak a negative behavior or find out to ride a bike without training wheels. The damaging stomach results and intense tachycardia generated by GLP1R agonists averts achieving the ultimate efficacy that might be accomplished with activation of GLP1R signaling. In a dose rise test of 2 doses daily, the topiramatedose was boosted biweekly by 16 mg to doses of 64, 96, 192, and 384 mg/d andthe resulting weight reduction were 5%, 4.8%, 6.3%, and 6.3%, specifically with theplacebo group losing 2.6%. The adverse occasions consisted of paresthesia, somnolenceand problem with memory, focus and focus such that 21% of thetopiramate groups took out due to adverse events [57] Topiramate growth as a medication for the treatment ofobesity was stopped Home page as a result of the adverse events.What is the new therapy for excessive weight?
Zepbound & #x 2122; (ZEHP-bownd) is an injectable prescription medicine that might aid adults with weight problems, or with excess weight (obese) that also have weight-related medical troubles, reduce weight and keep it off. It should be used with a reduced-calorie diet plan and increased physical activity.
Social Links