September 5, 2024
Weight Problems Medications In Advancement Pmc

What is the toughest weight loss medication?
What is the best weight management prescription drug? The amount of weight loss feasible with semaglutide, according to medical researches, is significant. A 2022 research study of 175 people showed 5.9% weight loss at 3 months and 10.9% at 6 months.

S6 Video Control Quiet-sleep
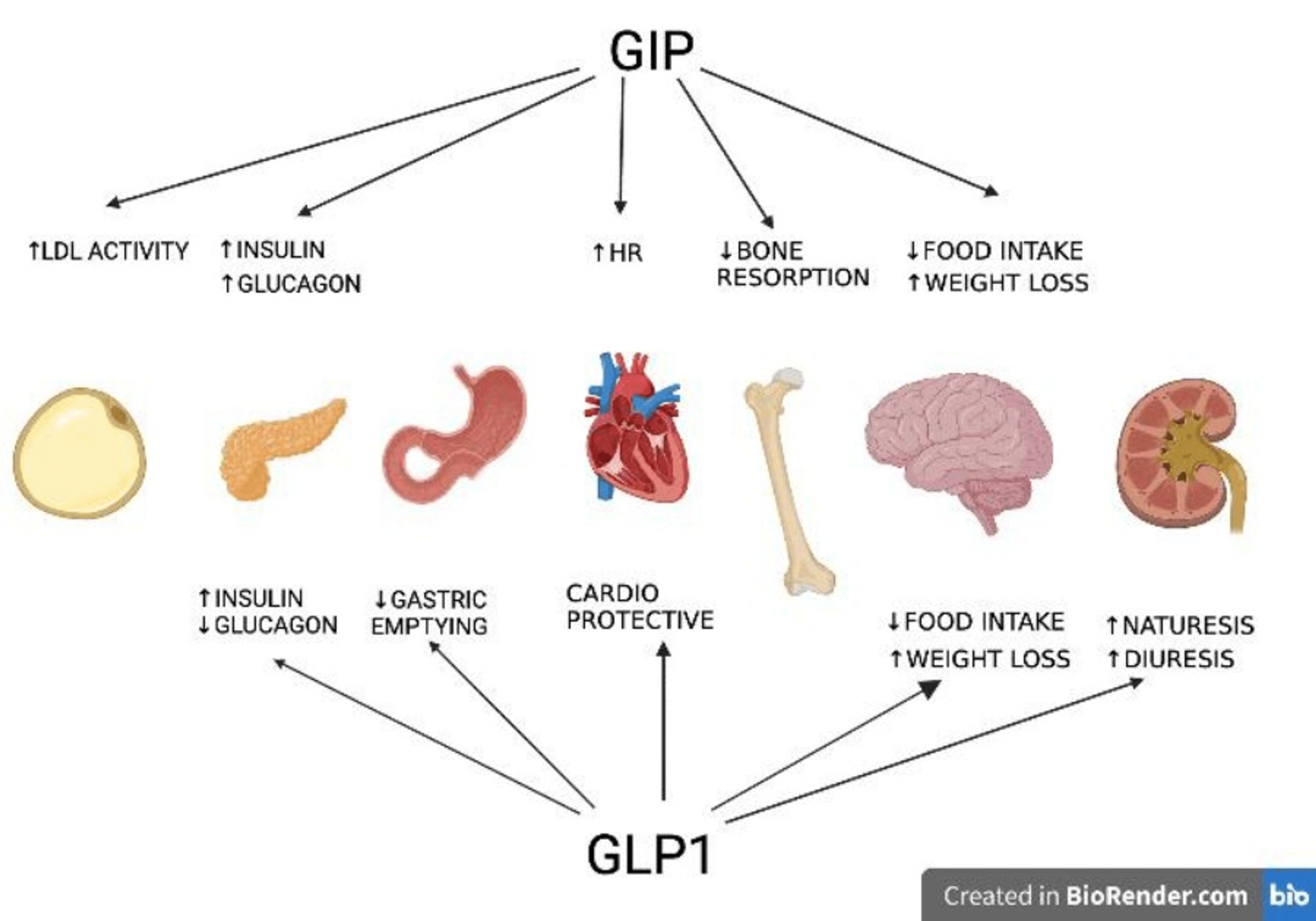
Since this drug combination has phentermine, it is a regulated medication enforcement administration (DEA) routine IV substance. Weight-loss medications produce an additional mean weight loss of only 3-- 5 kg above that of diet plan and placebo over 6 months, and more reliable pharmacotherapy of excessive weight is needed. We assessed the effectiveness and safety and security of tesofensine-- an inhibitor of the presynaptic uptake of noradrenaline, dopamine, and serotonin-- in patients with obesity. The quest of AOMs has been an enduring effort pushed over the last few years by a number of simultaneous growths. The most notable breakthrough in that instructions has actually been the discovery of poly-agonists that concurrently target the GLP1, GIP and/or glucagon receptors188,189. One of the most famous strategies pertain to unimolecular combination of GIP and/or glucagon receptor (GcgR) agonism with very potent, complementary GLP1R agonism. GIPR agonists, when chemically integrated with GLP1R agonism, have actually shown metabolic advantages and minimized body weight in mice when compared to pharmacokinetically matched GLP1R agonists122,189. There are several reasons why GIP agonism could provide supplemental metabolic advantages to GLP1 therapy, apart from reducing body weight and food consumption by means of GLP1R-independent mechanisms184,185.- Our data suggest that tesofensine in rats did not harm sweetness discovery or influence its palatability.
- Clinical application will continue and concentrate on loved one efficacy and safety, which is tough to refer when best-in-class prospects are at the same time swiftly progressing and not immediately obtainable for direct relative medical study125.
- Antibodies established with a lesser regularity in liraglutide-treated topics than in those treated by exenatide, likely because of its better architectural similarity with human GLP-1 (97 vs. 52%).
- As stated previously in area 2.3, a side effect brought on by thenon-specific serotonin agonists, fenfluramine and dexfenfluramine, was heartvalve lesions, because of excitement of the outer serotonin 2B receptor.
- Tesofensine Peptide may have different impacts on various people, yet it's best combined with a decreased calorie intake and routine workout.
Summary Of Tesofensine's Influence On Appetite Reductions, Metabolic Rate, And Fat Decrease
Nevertheless, these findings on the efficiency and safety and security of tesofensine with regard to its possible damaging effects (cardiovascular and CNS) require confirmation in phase III trials performed in bigger mates of overweight clients. Amylin produced by pancreatic β-cells acts to decrease post-prandial glucagon secretion, sluggish stomach draining, and centrally raise satiety [88] Very early studies revealed that pramlintide usage in people with insulin-treated diabetes boosted glycemic control and supported weight reduction by lowering food consumption [89] A succeeding research of pramlintide showed an added mean weight-loss of 3.7 kg vs. placebo in overweight clients without T2DM or with non-insulin-treated T2DM [89] While pramlintide monotherapy led to 1.5 kg additional weight-loss compared with placebo over 24 weeks, combination of pramlintide with either phentermine or sibutramine caused 9.2 kg weight-loss [90] Nonetheless, weight decrease with the medicine were unsatisfactory creating discontinuation in its growth [91]Assists With Weight Loss
Mean weight-loss was ~ 5%, with 15.5% of patients accomplishing fat burning above 10% about 5.8% obtaining liraglutide 1.8 mg. Body weight loss of ~ 7% was reported after 4 weeks of treatment, with improvements in glucose resistance. Whether additional unimolecular https://pharma-industry-ethics.b-cdn.net/pharma-industry-ethics/product/tesofensine-an-unique-antiobesity-medication.html GLP1R/GcgR co-agonists with better family member glucagon task or even more extensive period of action show much more effective, and adequately risk-free for persistent usage, stays to be determined202. In subjects with excessive weight, Licogliflozin (150 mg/day) therapy for 12 weeks resulted in a reduction in body weight by 5.7% (6.16 kg) compared to placebo which transcends to the impacts of SGLT 2 preventions. The gastrointestinal adverse events were more regular in the cured teams compared with the placebo, and enhanced with the dose.Social Links