
September 5, 2024
Part Three Next Generation Obesity Therapies
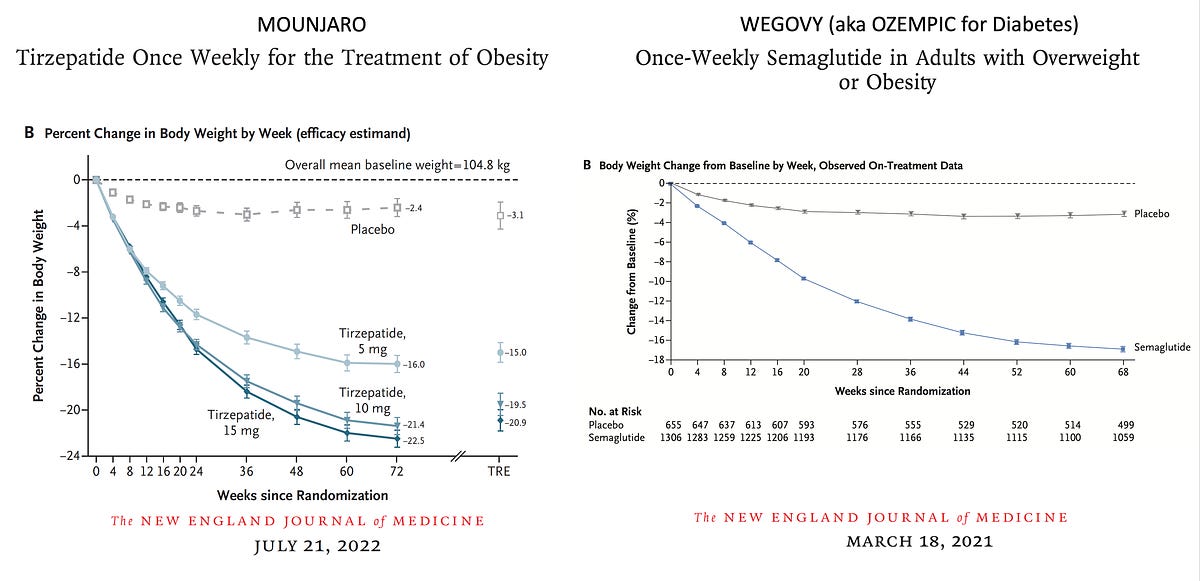
Excessive Weight Medicines In Development Pmc A noteworthy exception is the lately authorized GLP1R agonist semaglutide 2.4 mg, which in stage III professional trials lowered body weight in people with obesity or overweight without diabetic issues after 68 weeks of treatment by − 14.9% relative to − 2.4% in placebo-treated controls38. The hypothalamus is the centre of neuroendocrine policy of power homeostasis and hunger. Maldevelopment of, or damages to, the vital hypothalamic nuclei disrupts the coordinated equilibrium between energy consumption and expenditure leading, to quick and excessive weight gain.- If a predictive correlate between metabolic profiling and propensity to weight loss can be developed, this can have a profound impact on the future of medical care in weight problems.
- Postprandial GLP-1 secretion is decreased in diabetic person patients compared with nondiabetic people.
- With a deep understanding of integrative practical medicine and the complexities of weight problems, our professionals go to the center of the field.
Medical Weight Loss
Aminorex was amodification of the phenylethylamine foundation that raised the launch ofnorepinephrine in the main nerves and lowered hunger [10] From 1967-- 1968,. the prevalenceof primary lung high blood pressure was 20-fold higher than it was in the periodfrom 1955-- 1966 in those countries. Aminorex was gotten rid of from the marketin 1968 because of its organization with primary lung hypertension and by 1972the prevalence of key pulmonary high blood pressure had been up to the level priorto the release of aminorex [11] Thesymptoms of dyspnea, syncope and upper body discomfort fell back in many cases, however up tohalf of the individuals subjected were dead by 1980 [10] It was this experience that sensitized theobesity neighborhood to the danger of primary lung high blood pressure withanti-obesity drugs.What is the best fat loss drug?
What is the greatest weight-loss prescription drug? The quantity of weight reduction feasible with semaglutide, according to scientific researches, is substantial. A 2022 study of 175 individuals showed 5.9% fat burning at three months and 10.9% at six months.
Medications For Dealing With Weight Problems
While this research was performed on pets, the outcomes are assuring for prospective human applications. The efficiency of tesofensine in lowering body weight and controlling hunger, in addition to its safety and security account, makes it an amazing prospect for future scientific tests in people. Results from a professional test showed that weight reduction with tesofensine peptide was considerably greater over a six-month period than those achieved with any of the medications currently offered. Weight-loss depended on 10.6% in individuals, which was roughly two times the weight-loss created by medications presently approved by the US FDA for treating excessive weight. We utilize oral tesofensine peptide, the latest game-changing peptide created for the treatment of excessive weight, as one of our strategies. Tesofensine vs semaglutide are 2 different weight reduction help that have both been proven reliable in clinical tests. Heart disease, cancer, and stroke are the leading reasons of follow this link fatality worldwide, in recent years [1] These diseases belong to the "epidemic of obesity," among the significant international health and wellness problems [2] Specifically, lockdown actions to limit the transmission of coronavirus have adversely impacted a range of weight monitoring practices, consisting of exercise and healthy and balanced consuming. In a rat design of diet-induced excessive weight (DIO), tesofensine treatmentproduced durable weight loss gone along with by hypophagia. To recognize the neuralpathways regulating weight-loss and hypophagia, reversal of these results wasinvestigated using numerous monoaminergic receptor antagonists co-administeredwith tesofensine. Tesofensine significantly minimized food intake in the first 12hours of management in a dosage reliant manner, with a maximum effect after3 days. The hypophagic result slowly dissipated and returned to manage levelsby day 15, yet the reduction in body weight continued throughout of the 16day experiment. 
Social Links