
September 5, 2024
Anti-obesity Medication Discovery: Advancements And Obstacles Nature Examines Medicine Discovery
Tesofensine Understanding And References The distance of each neuron to the centroid of their corresponding cluster was after that determined. As the variety of sets enhanced, the distances to the centroid of each ensemble were decreased. A contour was then created by plotting the overall range within each ensemble against the number of ensembles checked.Relevant Terms:
Which drug is best for slendering?

Bupropion-naltrexone
While the medicine fell short to attain the key end factor of 5 percent weight loss contrasted to sugar pill, it did satisfy the FDA's specific effectiveness requirement. The portion of clients in the medicine team who lost a minimum of 5 percent of their body weight was 3 times that in the sugar pill group-- 55.6 percent to 17.5 percent at 28 weeks; longer trial arms showed comparable results. More important for repayment, the drug documented statistically considerable improvements in cardiovascular danger variables.- They suggested that the greater efficacy was as a result of the capacity of tesofensine to recover reduced DA levels in the center accumbens observed in overweight rats [3]
- Pfizer's antidepressant Zoloft (sertraline) is commonly suggested for short-term, off-label use, but obese people require help over the long run, and no long-term trials have been carried out.
- In 2018, the prevalence of weight problems, with a body mass index (BMI) of 25 kg/m2 or greater, among grown-up men and women was 45.4% and 26.5%, specifically.
- To conclude, theADVANS study provided some indicators of an antiparkinsonian task of the dopamine reuptake prevention tesofensine in advanced PD.
Medications Signed Up In Other Illness Entities Demonstrating A Weight-reducing Impact
Naltrexone ER/bupropion ER should be made use of with care in older people and is not suggested for those older than 75 years. Its pharmacokinetics in individuals with damaged liver and kidney feature have not yet been adequately examined. If naltrexone ER/bupropion ER is required for clients with damaged liver function, an optimum of one tablet per day can be provided and, in people with damaged kidney function, the optimum dose is two tablets daily. The drug is contraindicated in individuals with severe hepatic disorder or end-stage kidney failure [33] Furthermore, all COR medical trials https://s5d4f86s465.s3.us-east.cloud-object-storage.appdomain.cloud/pharmacovigilance/product-distribution/lasting-efficacy-and-safety-of-anti-obesity-therapy-where-do-we-st.html came along in cardiometabolic criteria, consisting of glycemic control, insulin resistance, and lipid accounts [28-- 32] We also made use of t-SNE to analyze the profile of motor results caused by appetite suppressants, in this case, clustering rats showing similar electric motor side effects. 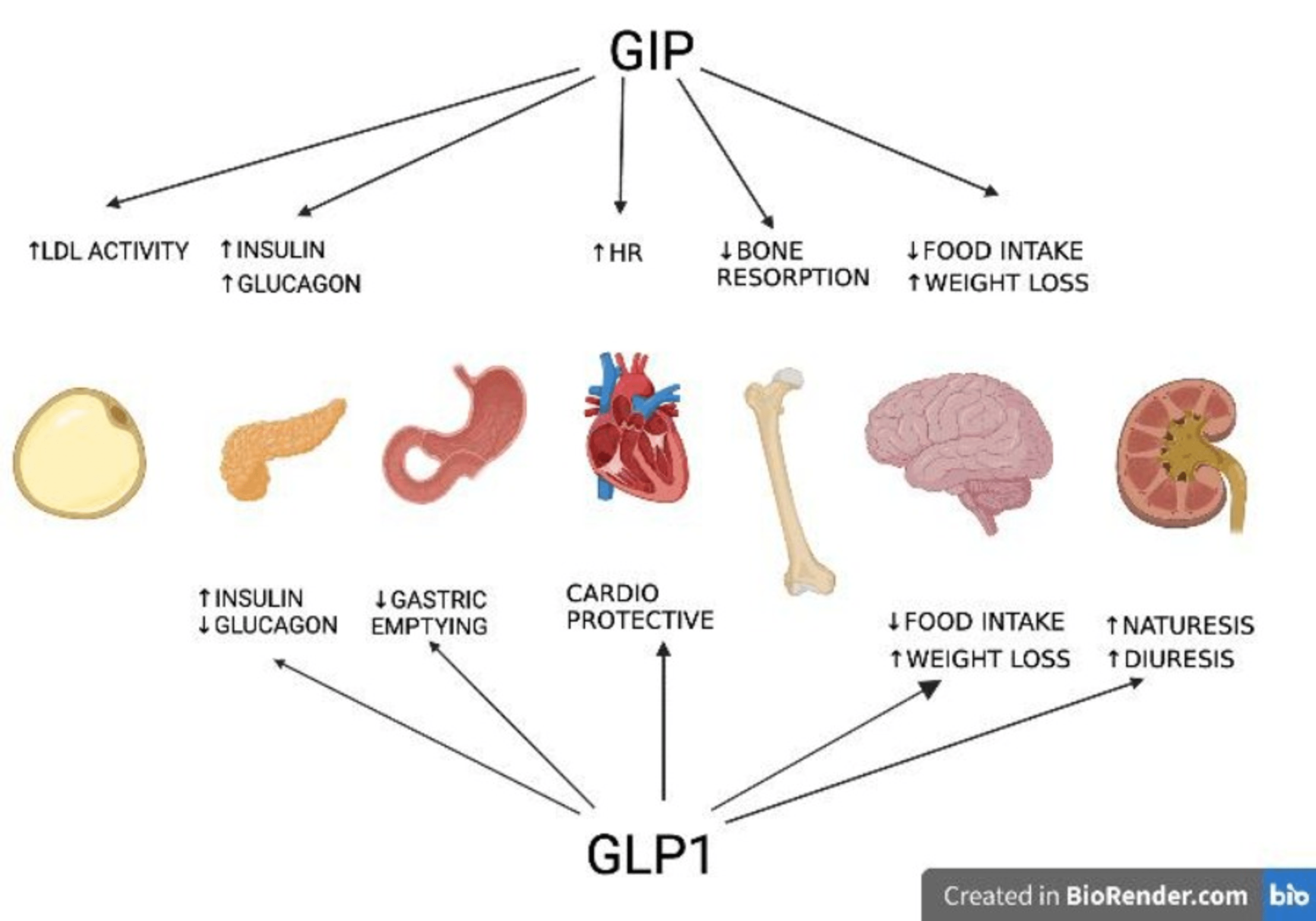
Social Links